
Search websites, locations, and people



ABOUT
ACADEMICS
RESEARCH
ADMISSIONS
NEWS & EVENTS
CAMPUS LIFE
INNOVATION
CAREERS
Imaging Tissues, Cells and Molecules: Westlake x Science Joint Online Symposium #7
14, 2023
Email: zhangchi@westlake.edu.cn
Phone: +86-(0)571-86886861
Office of Public Affairs
On June 29th, 2023, a large worldwide audience of 30,000 viewers enjoyed the seventh part of our live, online symposium series jointly organized by Science/AAAS and Westlake University, entitled "Imaging Tissues, Cells and Molecules".
The past decade has witnessed a revolution in our capability to image tissues, cells, and molecules - an achievement underscored by the awarding of two Nobel Prizes for super-resolution imaging in 2014 and cryo-electron microscopy in 2017. In the symposium, distinguished researchers, including Dr. Wolfgang P. Baumeister (Max Planck Institute of Biochemistry), Dr. Jennifer Lippincott-Schwartz (Howard Hughes Medical Institute), and Dr. Edward S. Boyden (MIT) presented key insights from their innovative research on these advanced imaging technologies and their associated discoveries. The symposium was co-chaired and facilitated by Dr. Yongdeng Zhang (Westlake University) and Dr. Stella Hurtley (Science/AAAS).
Research Highlights
The first speaker, Dr. Wolfgang P. Baumeister, is a pioneer in cryo-electron tomography, a technique widely recognized as having unique potential for visualizing molecular complexes in their functional cellular context and in situ structural biology. He talked about the power of seeing the whole picture by taking advantage of cryo-electron tomography.
Beginning with the introduction of cryo-electron tomography, Dr. Baumeister illustrated that this technique is the application of tomography principles in data acquisition and reconstruction, enabling high-resolution 3D imaging while preserving the in-situ structure of cells. Then he briefly reviewed the advances in electron microscopy (EM) technology in the past decade, the so-called 'resolution revolution', and pointed out that one of the critical factors was the automation of data acquisition: i.e., higher throughput with consistent imaging quality. One breakthrough of this period was the replacement of the traditional CCD cameras with direct electronic detectors, which, together with other devices such as phase plates, effectively improved the contrast of the images.
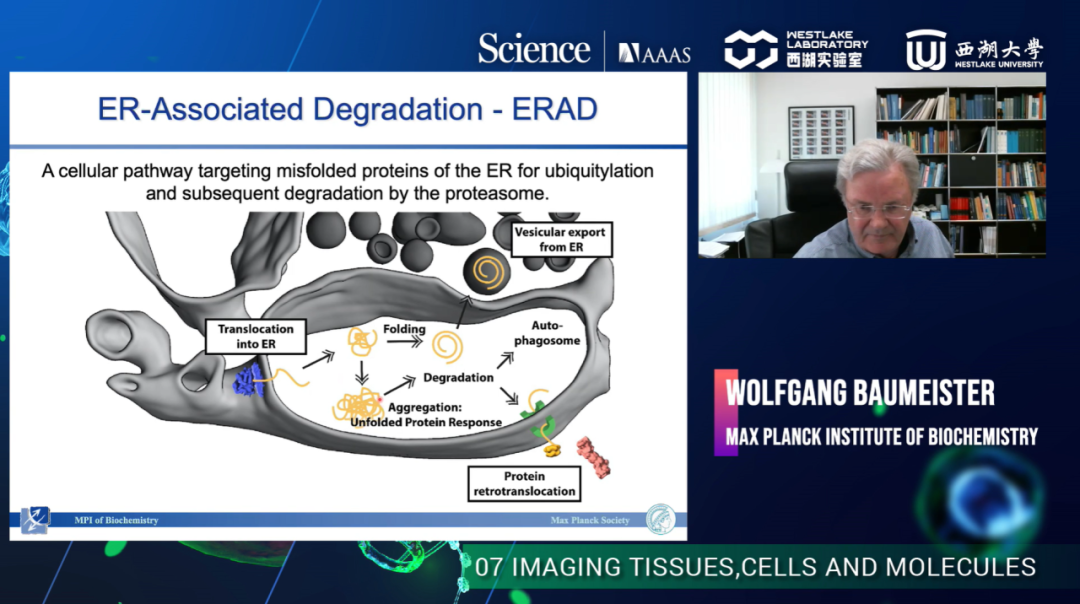
Next, Dr. Baumeister showed several examples resulting from all these technological advances. The first one is the observation of chaperonin complexes in the act of protein folding in vitro and in situ. They studied the GroEL/MetK complex with cryo-ET, and the tomogram of an E. coli cell displayed enhanced images from the outcome of sub-tomogram averaging. The second example is the 26S proteasome, which undergoes a huge scale of conformational changes during the functional cycle. The tomograms showed where the proteasome clusters were located. Under normal conditions, the majority was found in the nucleus, a major degradation compartment. Some clusters are also located in the endoplasmic reticulum and the Golgi apparatus. The sorted proteins were aligned and averaged by zooming into the clusters, resulting in a uniformly resolved subtomogram. Such molecule-by-molecule analysis of tomograms can further help infer which state the proteasome is in (ground state or substrate processing state). The third example concerns their recent research on toxic protein aggregation in neurodegeneration, such as Huntingtin protein and poly-GA aggregation. These two cases show that different aggregates are actually associated with different underlying mechanisms.
Finally, in terms of the future of the field, Dr. Baumeister said the question is how far we can push the resolution in situ, especially when it comes to smaller and less abundant structures. He believes there is no silver bullet, but there is still much that can be done to unlock the full potential of cryo-ET.
The next speaker was Dr. Jennifer Lippincott-Schwartz from HHMI. The topic of her report was “Looking under the Hood of Cells: from whole cell organelle reconstructions to single molecule dynamics to atomic reconstructions of macromolecules”.
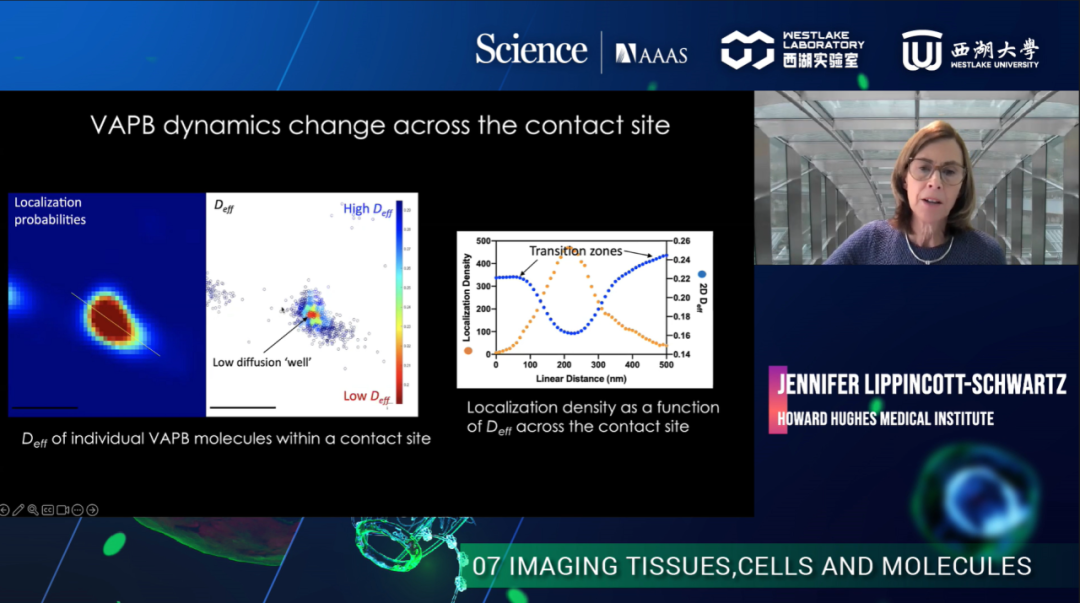
The first part of her talk covered the dynamic imaging of cells. Dr. Lippincott Schwartz's laboratory uses lattice light sheet microscopy to perform simultaneous multi-color 3D imaging of living cells to study the interaction between organelles. The results showed that the interaction between mitochondria and endoplasmic reticulum was the most significant among all organelle interactions. Next, they used single-molecule tracking to investigate the distinct mobility patterns of VAPB and its mutants at the ER-mitochondria contract sites in normal and amyotrophic lateral sclerosis (ALS) disease.
In the second part, Dr. Lippincott Schwartz talked about structural imaging. Dr. Lippincott Schwartz cooperated with other groups to develop a FIB-SEM technique that achieved 4-8 nm isotropic resolution in HeLa cells and constructed a whole-cell organelle database. However, FIB-SEM imaging lacks specificity and cannot be used to study organelles that lack structural features. Therefore, they developed cryo-structured illumination microscopy (Cryo-SIM) and FIB-SEM pipeline. Adding a cryo-super-resolution fluorescence imaging step before using FIB-SEM imaging, and combining the two results, can accurately locate specific sites and obtain electron microscope ultra-micro information through super-resolution fluorescence imaging results. They used this technique to study the mechanism of protein sorting at endoplasmic reticulum (ER) exit sites (ERESs) and revealed ultrastructural features at the region.
The final speaker, Dr. Edward S. Boyden, covered the topic of “Optical Tools for Analyzing and Repairing Biological Systems”.
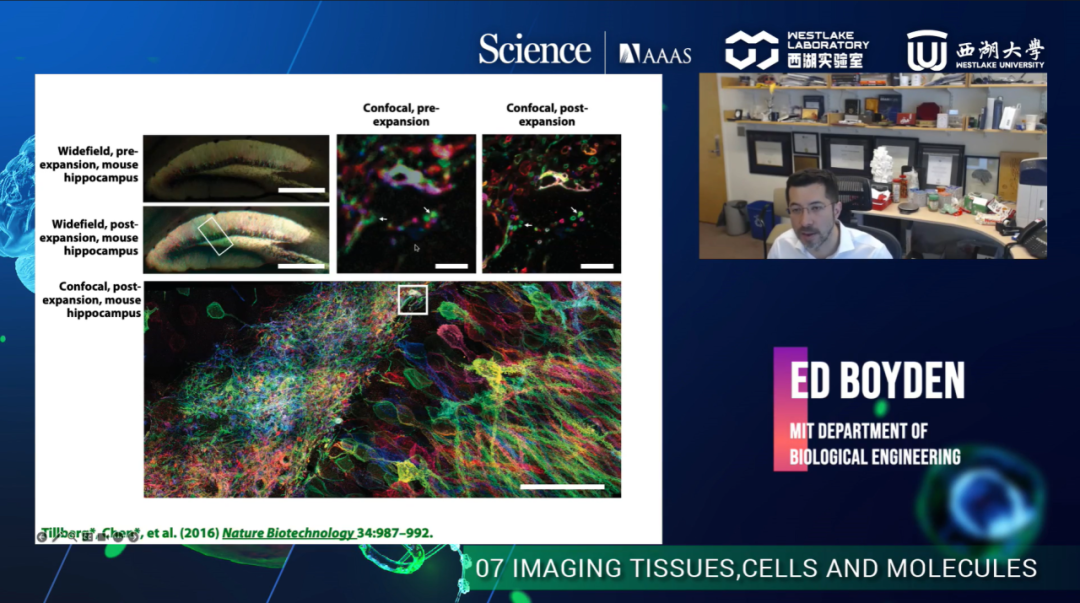
Dr. Boyden started by introducing Expansion Microscopy (ExM), a technique that improves the effective resolution of conventional microscopes via the physical expansion of biological samples. By covalently anchoring specific labels within the specimen directly to the polymer network, labels spaced closer than the optical diffraction limit can be nearly isotopically separated and optically resolved. In dense biological samples, the target molecules can be pulled apart with the extension of the long chain of the polymer gel. Initially, the method can improve the resolution by the expansion factor (~4.5), yielding a resolution of about 75 nm when combined with confocal microscopy. Moreover, it can also be combined with conventional confocal microscopy and lattice light-sheet microscopy to achieve super-resolution imaging of tissue samples, such as brain slices. Dr. Boyden described not only the group's work about a variety of biomolecules, such as proteins and RNA but also recent advances in ExM recipes that enable approximately 20-fold amplification of tissue samples (ExR). In recent years, ExM has not only improved the expansion factor but has also been applied to various complex samples to obtain more information.
Optogenetics integrates optics, software control, gene manipulation, electrophysiology, and other interdisciplinary technologies. The initial implementation of this technology is through the expression of light-sensitive proteins in cells to enable functional regulation based on the on-off response to light stimulation. Dr. Boyden described how three ion channels (H+, Cl-, and metal ions) selectively regulate the cellular excitability of ions. Due to the development of diverse photosensitive proteins, various photo-regulatory functions can be explored in living cells. To analyze how a signal transduction network converts cellular inputs into cellular outputs, ideally, one could simultaneously measure the dynamics of many signals in the network. Dr. Boyden found that fusing the fluorescent reporter protein to a pair of self-assembling peptides can stably cluster at random points within the cell at distances far enough to be resolved by a microscope but close enough to sample the biology of interest in space. Because such clusters, called signaling reporter islands (SiRIs), can be designed modularly, they permit a set of fluorescent reporters to be efficiently adapted to simultaneously measure multiple nodes of a signal transduction network within single cells. They were used in cultured hippocampal neurons and intact brain slices to discover the relationship between the speed of calcium signaling and the amplitude of PKA signaling upon receiving a cAMP-driving stimulus.
The symposium concluded with an open Q&A discussion section whereby insightful and innovative ideas were shared between the speakers and co-chairs. To enjoy the full playback and open discussion of ‘Imaging Tissues, Cells and Molecules’ jointly organized by Science/AAAS and Westlake University, please visit: https://live.vhall.com/v3/lives/watch/418528741.
We would like to thank the three distinguished guest speakers sincerely, Dr. Wolfgang P. Baumeister, Dr. Jennifer Lippincott-Schwartz, and Dr. Edward S. Boyden, for their time and openness in sharing their latest research and ideas in the field of imaging, as well as co-chairs Dr. Yongdeng Zhang and Dr. Stella Hurtley for their expertise and assistance. We would also like to sincerely thank all the audience who helped facilitate an informative and engaging symposium worldwide.
Please check out our previous parts of this symposium series, and we very much look forward to you joining us in our upcoming parts as we work towards an open and global platform for scientific discussion and innovation.
Science/AAAS and Westlake University Symposium Series
Part 1 | Gene Editing | https://live.vhall.com/v3/lives/watch/925591016
Part 2 | Biomolecular Condensates | https://live.vhall.com/v3/lives/watch/340760384
Part 3 | Protein Engineering | https://live.vhall.com/v3/lives/watch/537973129
Part 4 | Dynamic Molecular Systems | https://live.vhall.com/v3/lives/watch/703086320
Part 5 | New Insights into Host–Virus Interactions | https://live.vhall.com/v3/lives/watch/123859990
Part 6 | Optogenetics | https://live.vhall.com/v3/lives/watch/957350315
Part 7 | Imaging Tissues, Cells and Molecules | https://live.vhall.com/v3/lives/watch/418528741
Part 8 | Mechanobiology | https://live.vhall.com/v3/lives/watch/166361837





















